Endoss is ready for the future
Standardization in the medical- and healthcare sector is on the rise. Endoss, supplier of disposables that improve patient safety, prefers to take a proactive approach rather than wait for Governments or Healthcare institutions to implement standardization requirements.
“We are investing in labels meeting the GS1 Standards right now, because not only can our customers benefit from them in the future, so can we. GS1 introduced us to Mediclabel by Type 2 Solutions. They helped us redesign our labels and incorporate a datamatrix. We’re ready for the future”, say Natasja Kuipers and Vincent van der Meer at Endoss.
UDI Datamatrix barcodes
A lot of hospitals are currently encouraging their suppliers to provide high-risk classification products, such as implants, with a GS1 label. It is only a matter of time before they will ask their suppliers to also label low-risk products, such as disposables, with a standardized barcode.
Back in 2016, Endoss started an international survey with its customers and distributors on how it should tackle the standardization of its product identification. Based on the response, a project was started which resulted in the application of UDI datamatrix barcodes on various articles – not only on item level, but also on box and master carton level.

ADC and MDR, based on GS1 Standards
Multiple international legislations, such as UDI and MDR, are about to be implemented in Europe. These laws oblige the suppliers of medical devices to supply their products with a Unique Device Identifier (UDI). A British distributor was the first to ask Endoss for standardization. This distributor was, in turn, compelled by the National Health Service (NHS), with a deadline of October 2017. In England, the choice was made to implement these laws using the GS1 standard for barcodes, because GS1 is the accredited agency for UDI identification.
Nastasja Kuipers, international account manager: “We created a graduation project out of it. Michael Raats, studying MER at the AVANS Hogeschool, wrote a clear Thesis that we could implement directly. Amongst other things, he researched in different countries and at our clients how a supplier could best implement the standard.”
Kuipers and Vincent Van der Meer (Quality Assurance & Regulatory Affairs Specialist) emphasize that Endoss chose to implement the GS1 Standard. They aim to comply to the new impending legislations such as the ADC (Agreements Unique Coding) and MDR (Medical Device Regulation).
Mediclabel software
Prior to the standardization project the supplier was mostly looking for answers to questions such as ‘what kind of standardization solutions are available’, ‘what requirements are being set by the market’ and ‘how do we switch to standardized deliveries’.
Van der Meer: “Afterwards we called in external help for generating the correct barcodes and data on our packaging. We couldn’t generate the datamatrix ourselves but met Type 2 Solutions through GS1. With their help, our labels were redesigned, and we use their Mediclabel software to generate the datamatrix barcodes.”
“In two years, we will be happy that we started standardizing early”
Endoss now supplies all products with GS1 compliant datamatrix barcodes. This Unique Device Identification will be legally required all over the world. International authorities are striving to use this identification to make medical devices easily traceable.
The International Medical Device Regulators Forum, the United States Food and Drug Administration and the European Commission are the ones who drive this legislation.
A tip for companies that are feeling the increasing pressure to standardize
Endoss is ready for the ADC to become law but does not wish to stand still the coming time. Kuipers and Van der Meer have a few tips for businesses that are feeling the pressure to standardize:
“Don’t wait, it will take a while before you have everything sorted out. It also helps to take a proactive role. Hospitals already have so many obligations, if you as a supplier can make it easier for them, then you should want that.”
Source: GS1
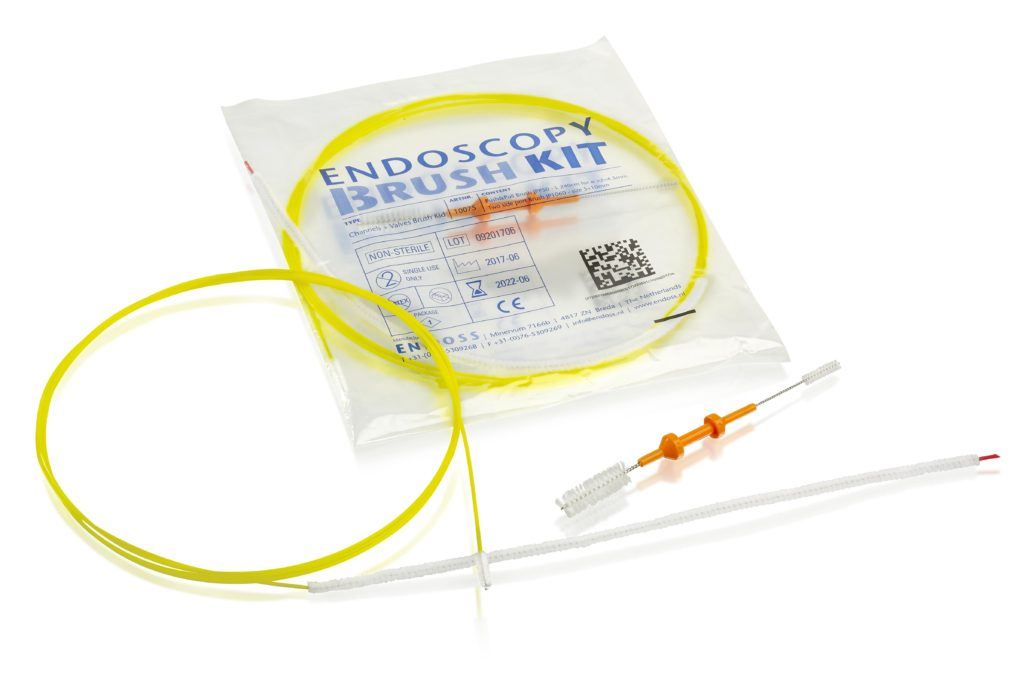
GS1 DataMatrix label created with the Mediclabel software of T2S.
GS1 Solution Provider
For more than 21 years, T2S has been supporting organizations with the implementation of the GS1 standard with software and expertise.
Would you like to know more?
Contact us or leave your contact details and we will call you back.